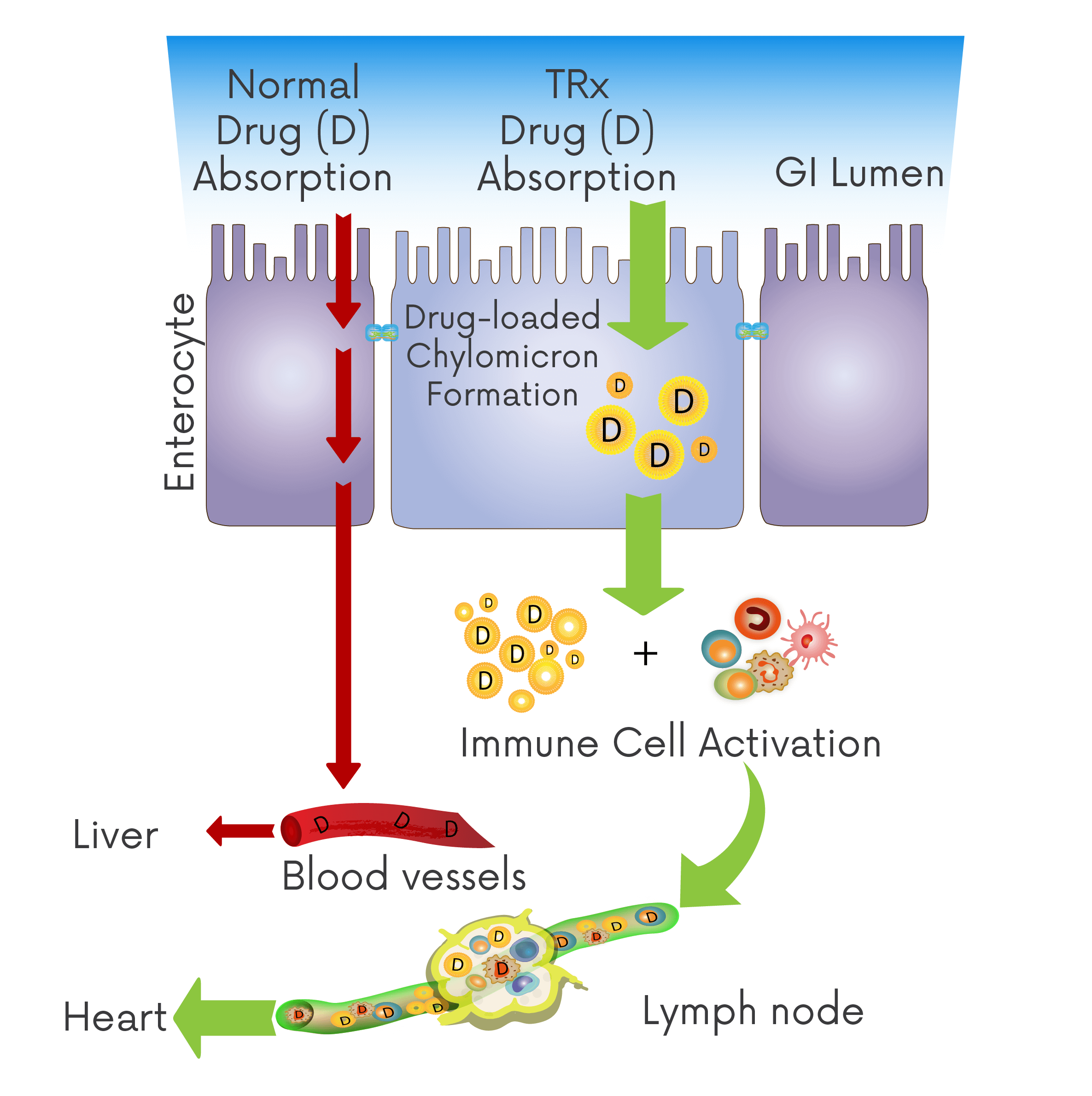
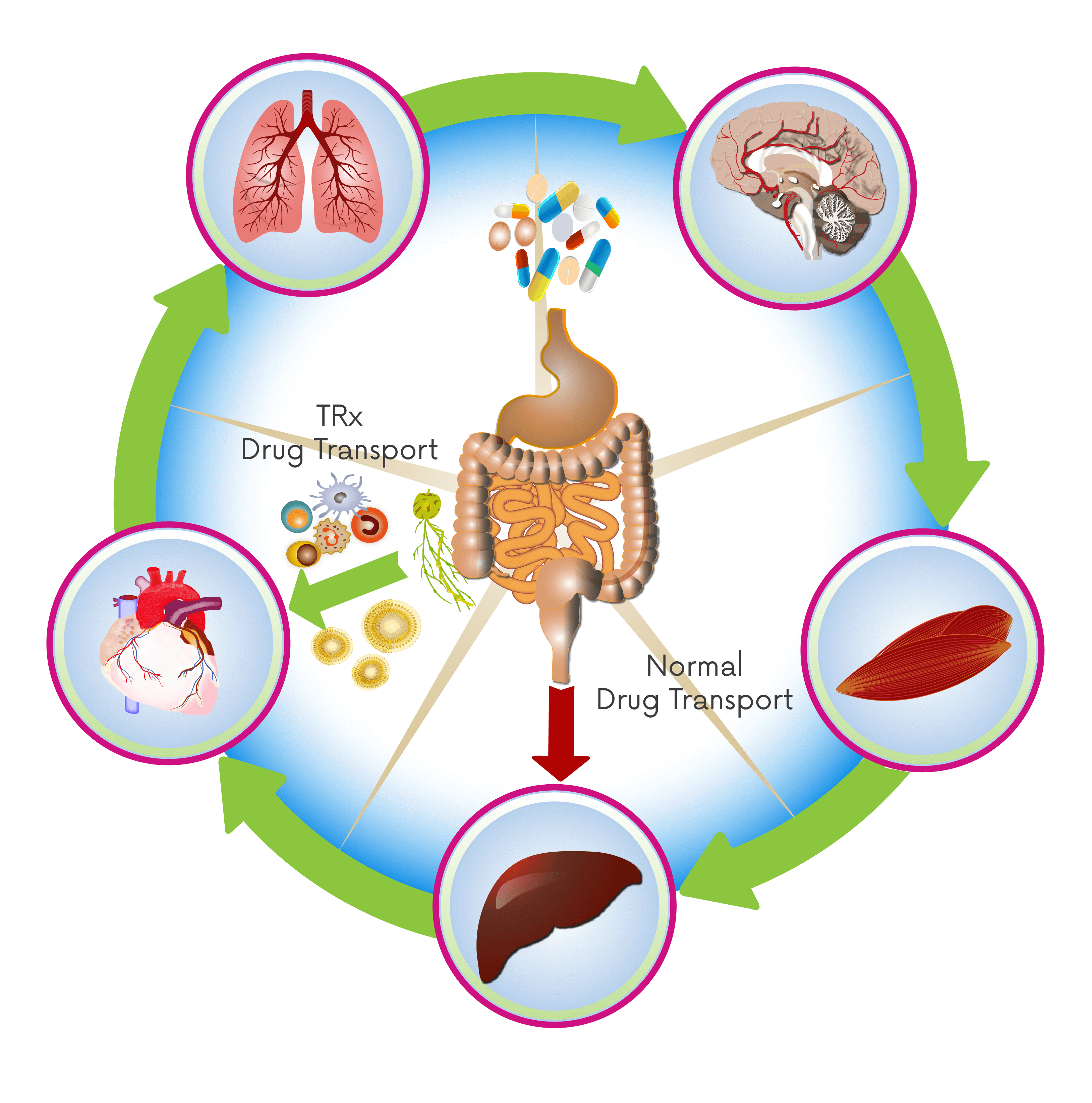
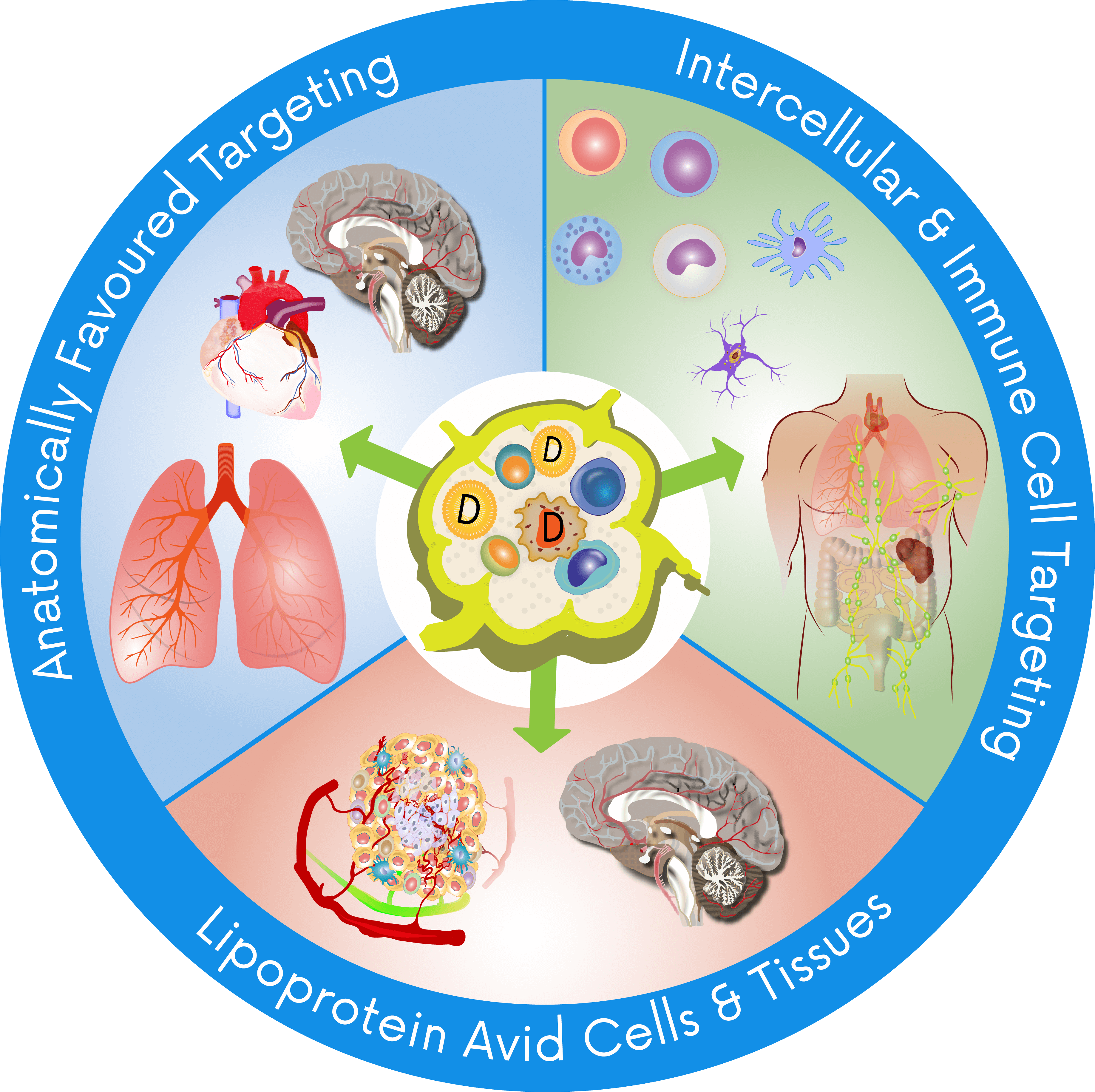
Radical approaches to drug absorption, transport and destination.
A disruptive advancement in oral drug uptake, trafficking therapeutics directly to the site of disease, reducing the impact of first-pass metabolism and enhancing production of regulatory immune cells. TRx is leveraging these and other benefits across a growing portfolio of co-development and wholly-owned programmes.